ALERT MANAGEMENT
In accordance with Directive 2011/62/EU of the European Parliament and of the Council amending Directive 2001/83/EC on the Community code relating to medicinal products for human use, as regards the prevention of the entry into the legal supply chain of falsified medicinal products and Directive 2011/62/EU - also called the Falsified Medicines Directive (FMD) individual national states are requested to set up and manage repository system connected to European verification systems to enable to identify and verify the authenticity of an individual pack of a medicinal product.
Czech medicines verification system (CZMVS) is provided as a “blueprint solution” by Solidsoft Reply managed by Czech medicines verification organisation (NOOL).
Article 37 of Delegated regulation defines the obligations of legal entities setting up and managing repository system on national level as part of European repository systems. This Article, among other things, obliges NOOL to ensure that all potential cases of counterfeiting identified in the system are immediately investigated in accordance with Article 36 (a) and, where counterfeiting is confirmed, notify the competent national authorities, the European Medicines Agency and the Commission.
Beginning in 1st January 2021, the "transitional period" ended, in which the Ministry of Health of the Czech Republic allowed to dispense medicinal products despite an error message, the so-called alert, which shows that the authenticity of the medicinal product was not successfully verified in NSOL. From this date, therefore, each alert needs to be checked and closed, more precisely it must be confirmed by the MAH or end user (or NOOL for system alerts) that the pack is not a counterfeit. Only then can the pharmacy dispense the medicine to the patient. Rapid and accurate investigation of the resulting alerts is therefore in the interest of all parties - marketing authorization holders, distributors, pharmacies and especially patients.
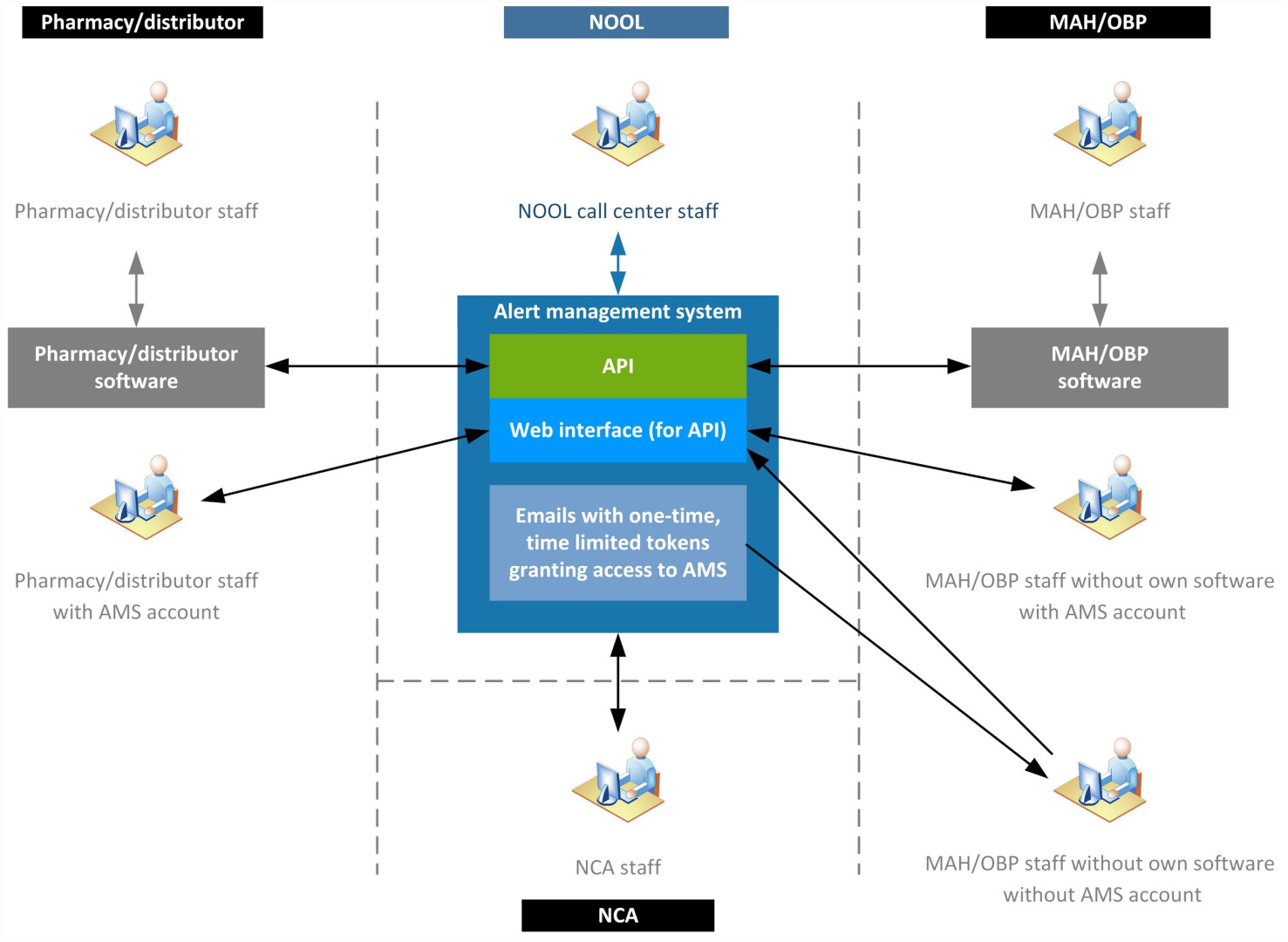
NOOL offers the Czech Alert Management System (CZAMS), where it is possible to quickly and conveniently resolve alerts via an API or web interface.
An CZECH ALERT MANAGEMENT SYSTEM was created by NOOL for the purpose of managing and evidence of investigation of individual alerts, as a support system for the Czech medicines verification system (CZMVS). The purpose fo this support system is to facilitate the administration associated with alerts investigations and to help automate the entire investigation process. Ultimately, the system helps to reduce the number of “false” suspicions of possible counterfeit.
Alerts can be managed in three ways:
- Web interface of the Czech Alert Management System.
- Integration of own user alert management system with Czech Alert management system using API communication.
- One-time, limited access to Alert Management System (for MAH/OBP, without registration in CZAMS)

 Registration and login to systems
Registration and login to systems











