Alert management system available for pharmacists and distributors
Dispensing despite an alert has ended. Now what?
The National Organization for the Authentication of Medicines (NOOL) has developed a unique Alert Management System (AMS), which aims to facilitate the investigation and resolution of alerts in a predefined form and in an interactive environment.
What is the Alert Management System?
This is a support system of the national system for verifying the authenticity of medicines that is managed by the National Organization for the Authentication of Medicines (NOOL). The purpose is to facilitate the administration associated with the investigation of alerts and the automation of the entire investigation process. The Alert Management System for marketing authorization holders (MAH) has been in operation since February 2019, continues to improve, and will be modified based on feedback from end users who are starting to use it now. The Alert Management System can be used through one-time API1) queries from end user systems or through the Alert Management System web interface.
What was the purpose of creating the Alert Management System?
- The system supports the investigation of alerts and makes the results available to end users.
- The system enables direct two-way anonymous communication between end users (pharmacies or distributors) and manufacturers (or marketing authorization holders – MAH) when investigating alerts, without the need for NOOL support center staff to mediate this communication, which speeds up the investigation of alerts.
- The system eliminates language barriers during communication between the MAH and end user.
For automated message generation it is essential that both sides, the end user and MAH, use the functionality
How the Alert Management System works
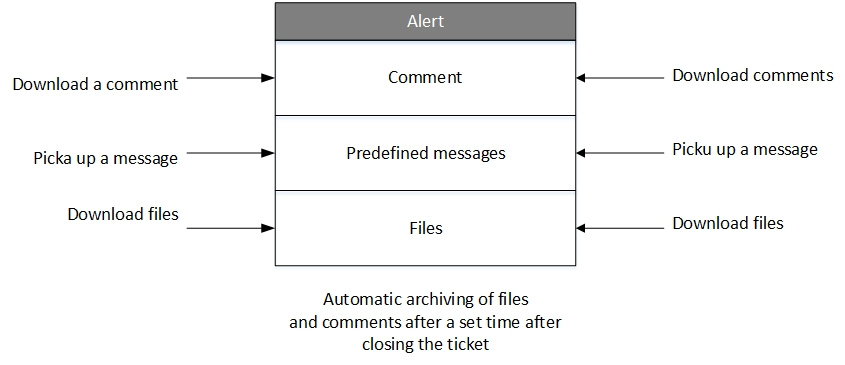
Frequently asked questions:
Why should our pharmacy connect to the system?
It is easier to determine the status of alerts, to obtain and enter more precise information for a given alert, and to communicate anonymously to resolve alerts with the MAH.
The Alert Management System also makes it possible for pharmacists to release quarantined medicinal products so they can be dispensed to patients after an alert is resolved and the medicine packaging is subsequently successfully verified. Prompt determination of alert status also prevents possible rejection of medicinal products being returned due to errors caused by end users, or if alerts originating on the MAH side are eliminated within the 14-day quarantine. On the other hand, MAH errors that cannot be corrected or alerts resolved after 14 days will enable arrangements to be made with the distributor to return goods within the standard procedures.
Important warning:
The Alert Management System does not by itself provide end users any information on whether they can or cannot dispense a given medicinal product. After closing an alert (eliminating the reasons it was triggered) it is necessary to again verify the unique identifier – with the exception of medicinal products confirmed to be exempt by the Ministry of Health of the CR pursuant to Section 11 letter r) of the Pharmaceuticals Act or medicines where the MAH has confirmed manufacturing release before 9.2.2019.
How can we connect to the Alert Management System?
If an end user would like to use the web interface to access the Alert Management System, they must contact NOOL to obtain login information – name (login) and password – at the following e-mail address: registrace@czmvo.cz
Can multiple logins be requested?
Yes, upon request NOOL can provide an end user with multiple login names and passwords.
What is the address of the alert management web interface?
How can the status of an alert be determined once?
If enabled by your application (communicating via one-time API1) queries), it is only necessary to enter the ID of the location/facility into the pharmacy or distribution system being used to verify medications.
Where can I find the facility/location ID?
This can be determined after logging in to the NSOL portal at https://portal-cz.nmvo.eu/, where you will find all registered locations pertaining to the “Organization”. To view details of each location including the location ID (a long text string generated automatically by NSOL), click the pencil icon on the right. The easiest way to determine the location ID is contacting support@alert-czmvo.cz. Please include your IČO (CIN) in the e-mail body and eventually the name of the organization. In cases where multiple locations are registered under a single organization, the person authorized to investigate alerts in terms of FMD will receive IDs for all related locations; pharmacy managers will only receive the ID of their location.
Is it possible to connect to the alert management web interface through the NOOL website (www.czmvo.cz)?
No, access through the NOOL website is only used for primary registration to NSOL and concluding contracts with NOOL.
Why do I need a facility/location ID?
For one-time queries regarding the alert status or if end user software requires/enables direct access to the Alert Management System web interface by using the facility/location ID.
What should software developers know?
- An API testing environment with full data set is available.
- NOOL will send login information upon request (registrace@czmvo.cz).
- IT development chooses a testing company (one only, can be changed), software modifications in internal applications can be debugged based on the alert data of this company.
- IT development will modify the client software to function with API1) according to specifications.
- After the software is debugged it can be connected to the production system.
API technical documentation on the NOOL website
Czech: https://www.czmvo.cz/file.php?id=441
English: https://www.czmvo.cz/file.php?id=445
- Application programming interface

 Registration and login to systems
Registration and login to systems












